
We will discuss the relationship between intermolecular forces and physical properties later, but for now, keep in mind that the stronger these interactions the higher the melting and boiling points are. In other words, there is not really such thing as one bond between two ions/atoms like in covalent compounds and that’s why they need a lot of thermal energy to break these interactions and therefore, have high melting points. Unlike the covalent bonding, ionic bonds are not directional and specific between given atoms, but rather they form a lattice as an interaction among the entire cluster of the ions: What is important here is that we are not talking about the interaction of one Na + and Cl + ion together. Among other interesting features and applications, we learn that it has a melting point of 801 ☌ which is extremely higher than the typical range for organic (covalent) compounds.

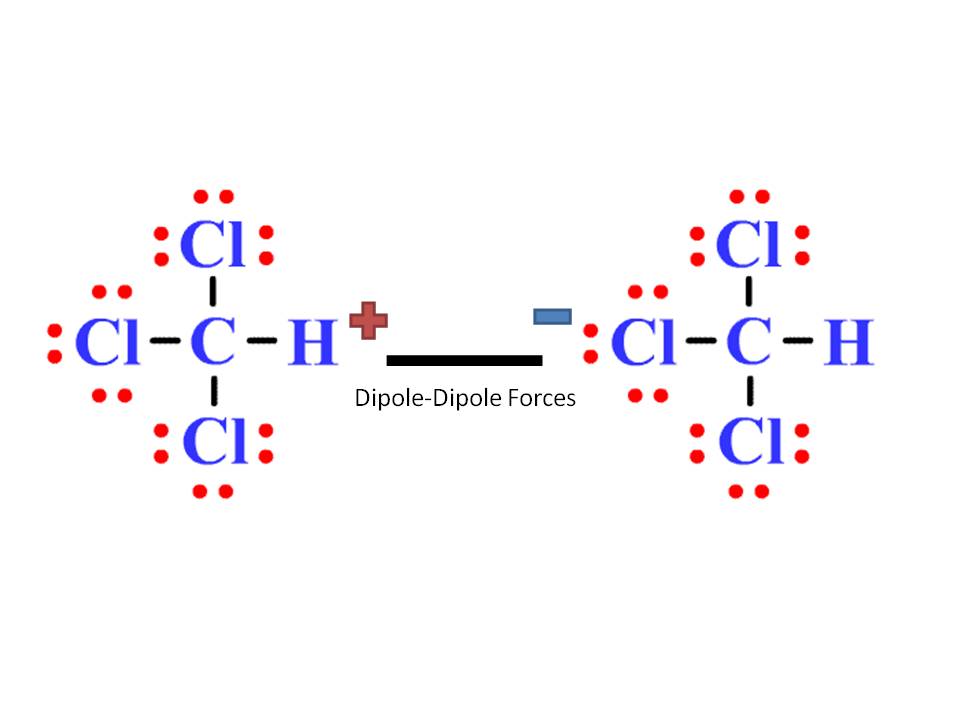
For example, sodium chloride (NaCl) is one of the first ionic compounds we talk about in general chemistry. All of them are electrostatic interactions meaning that they all occur as a result of the attraction between opposite charges and which of these forces is present or predominates in a given compound, depends on its functional groups.īack from general chemistry, we know from the structure of salts that oppositely charged particles tend to show very strong electrostatic interactions and these are the ionic compounds. Dipole-dipole, London dispersion (also known as Van der Waals) interactions, hydrogen bonding, and ionic bonds are the main types of intermolecular interactions responsible for the physical properties of compounds.


 0 kommentar(er)
0 kommentar(er)
